Serious diamond connoisseurs consider a diamond’s 4Cs as essential information. Some buyers might also want to know about the diamond type. But what exactly does diamond type mean?

Left to right: 0.47 ct type Ia pink; 0.38 ct type Ia “cape” yellow; 1.04 ct type IIa colorless; 0.56 ct type IIb blue; and 1.01 ct type Ib “canary” yellow.
Diamond type is a simple way of classifying diamonds based on their color and physical properties. It is important because it helps to form the basis of the identification of natural, synthetic and treated diamonds. Diamonds are composed of essentially pure carbon. However, many diamonds also contain trace elements, such as nitrogen or boron, acquired naturally during the course of formation.
These simplified diagrams show how nitrogen and boron atoms can replace carbon atoms in the diamond lattice.
In other diamonds, trace elements are added as the result of treatment or synthesis in a laboratory. They also contain other imperfections in the lattice of carbon atoms – scientists refer to these imperfections as lattice defects or optical defects. These trace elements and other imperfections are important because they cause the color and ultraviolet fluorescence reactions in diamond. The presence or absence of these lattice defects, their amounts, and their arrangement within the lattice can affect a diamond’s appearance, sometimes in dramatic ways.
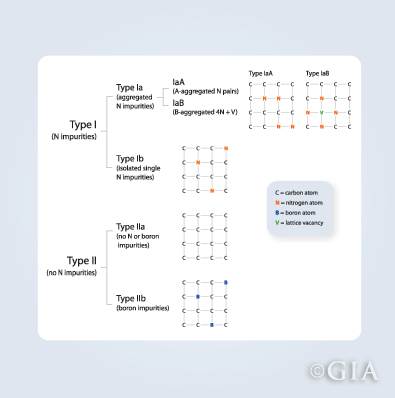
In the 1930s, scientists first began to use two categories to describe a diamond’s chemical composition and atomic structure: type I and type II. Now these categories are further subdivided into type Ia, type Ib and type IIa and type IIb.
Type Ia:
These diamonds contain nitrogen atoms in clusters. Approximately 95% of natural diamonds are type Ia. They normally vary from near-colorless to light yellow. These so-called “cape” diamonds derived their name from diamonds that were initially mined in or near Cape Province, South Africa.
Type Ib:
These diamonds also contain nitrogen, but as isolated atoms instead of clusters. They are often bright yellow in color, and are extremely rare. The trade sometimes refers to these colors as “canary.”
Type IIa:
These diamonds have no measurable nitrogen or boron impurities; they are usually colorless but they can also be gray, light brown, light yellow or light pink. Among all diamonds, those that are type IIa are chemically the most pure.
Type IIb:
These diamonds are known to conduct electricity. The trace element boron is responsible for most diamonds that are blue or grayish blue in color.

The historic Wittelsbach Blue diamond sold for a record-breaking $24.3 million to London jeweler Laurence Graff at Christie’s in December 2008. It was subsequently recut to 31.06 ct, as shown here, and renamed the Wittelsbach-Graff.
Knowledge of diamond type is important for gemologists because it allows them to help determine if a diamond is natural, synthetic or treated and whether it should be sent to a laboratory for further testing.

GIA uses an FTIR spectrometer to determine diamond type. The absorption spectrum identifies the diamond’s type.
GIA offers Diamond Type Analysis as a supplemental service to GIA diamond reports. GIA uses a Fourier transform infrared (FTIR) spectrometer an instrument which measures the absorption of electromagnetic radiation of the diamond. The result is an absorption spectrum from which diamond type can be determined.

When a diamond arrives at a GIA lab, it is tested on a FTIR spectrometer. This is one part of the identification process. For example, a type IIa may be natural, synthetic, or treated.
Considering a diamond purchase? Then be sure you understand the basic 4Cs. But if you’re looking to take your diamond knowledge to another level, then learning about diamond types is a good place to start.
Main Image of The Millenium Star (pictured above) is classified as a type IIa diamond.




